Fuel Cells: A Unique History
A historical overview of fuel cell technological development from 1800 to the present day
Introduction
In the year 1800, most of the world’s energy was produced by burning wood or coal. Houses in Europe were heated with wood-burning fireplaces, the first trains were beginning to run on steam power, while horse-drawn carriages were still popular throughout the towns and cities.
At the same time, however, the precursors to today’s cutting-edge fuel cells were being developed. While these initial experiments were simple by today’s standards – they form the basis of all contemporary fuel cells and their impact on science and technology has been significant. From powering space flight to providing clean energy for hydrogen cars, early research on fuel cells has contributed to some of the most remarkable breakthroughs of the last century.
This article will give a complete history of fuel cells and fuel cell stack development – taking you through the key discoveries, innovations, and applications over the last 200 years.
Early Beginnings: Anthony Carlisle and William Grove, 1800-1842.
While they didn’t invent a fuel cell, Anthony Carlisle and William Nicholson are usually credited with creating the first-ever electrolyzer. In 1800, they passed an electrical wire through water and were shocked at the result:
“Mr. Carlisle observed a disengagement of gas round the touching wire”, Nicholson recalls in his notebook. “This gas, though very minute in quantity, evidently seemed to me to have the smell afforded by hydrogen”.
After a few further experiments, Carlisle and Nicholson were able to perceive two separate streams of bubbles coming out of the metal wire they passed through water: “It was natural to conjecture, that the larger stream was hydrogen, and the smaller oxygen”, wrote Nicholson.
His speculation turned out to be correct – and Anthony Carlisle and William Nicholson had just created the world’s first electrolyzer.
It was British physical scientist William Grove, however, who finally invented the first fuel cell four decades later. In 1842 Grove produced what he called a ‘gas voltaic battery’, a device that combined hydrogen and oxygen to produce electricity.
Grove wrote that his device was "composed of alternate tubs of oxygen and hydrogen” that had a foil “dip into the separate vessels”. After 60 of these alternations, the foil gave him an “unpleasant shock”.
While Grove’s fuel cell produced enough electricity to shock him, the electricity created wasn’t enough to be commercially viable – and it wouldn’t be for another five decades that fuel cell technology would see another big advancement.


The First Breakthroughs, 1889-1932
In 1889, two British chemists, Ludwig Mond and Charles Langer, developed a device that produced much more electricity than Grove’s invention while also having a longer service life. More power output was achieved by using electrodes of thin, perforated platinum – while the reliability was improved by making the electrolytes semi-solid.
All this enabled their invention – which for the first time they called a ‘Fuel Cell’ – to produce more electricity than the previous generation (producing up to 6 amps per square foot).
This was followed by other breakthroughs: Baltic-German chemist Friedrich Ostwald provided a theoretical understanding of the different components of fuel cells in 1893, American electrical engineer William Jacques constructed the first carbon battery in 1896, and Swiss chemist Emil Baur experimented with different types of electrolytes (made out of molten silver, clay and metal oxides) in the early 1900s.
Towards a Practical Fuel Cell: The “Bacon Cell”, 1932-1959
Beginning in 1932, English engineer Francis Bacon started on a project that would, for the first time, make fuel cells practical to use in real-world applications. Up until now, fuel cells were considered a mere scientific curiosity. Bacon, however, was convinced of their usefulness – conceptualizing a simple, economical design that was capable of producing much more power than ever before. This eventually became the world’s first alkaline fuel cell (AFC).
Between 1932 and 1941, Bacon worked alone experimenting with an alkaline electrolyte design, replacing expensive metals used as catalysts with nickel. From 1941 to 1961, Bacon’s success allowed him to organize several research teams throughout Britain that refined, updated and modernized the design. The result was a system that came to be regarded as the ‘benchmark’ of fuel cell design: the Bacon Cell.
With the six-kW version unveiled in 1959, the Bacon Cell set the standard for fuel cell technology – being powerful enough to be used in real-world applications for the first time.

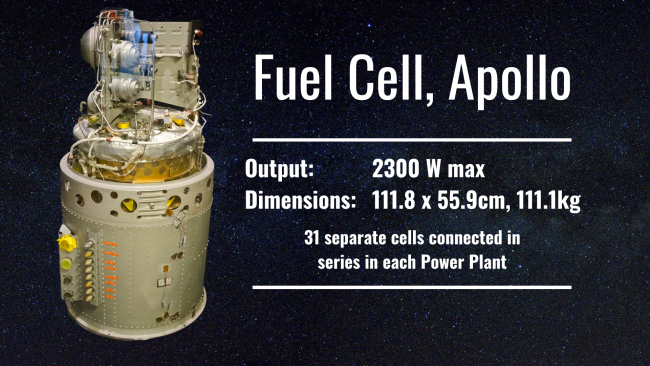
NASA, The Space Race, and Apollo Missions: Fuel Cells in Spaceflight, 1961-1981
Bacon’s fuel cell came of age just in time to be used in an exciting application: spaceflight. When NASA’s scientists were searching for a light, powerful and efficient solution to power Apollo spacecraft, Bacon’s fuel cell design ticked all the boxes.
It was lighter and much less bulky than batteries of the time, it was much more efficient than 1960’s solar panels, and the key elements needed for operation (hydrogen and oxygen) were already going to onboard the spaceship as rocket fuel. Not only this, but the only waste that fuel cell produced was pure water – perfect for the astronauts to drink.
“Bacon’s fuel cell was a tremendous achievement,” said Professor John Davidson, former Head of Department at Cambridge University’s Department of Chemical Engineering and Biotechnology. “The result of a lifetime’s hard work, persistence and ingenuity.”
Francis Bacon was even invited to meet President Richard Nixon in 1970, where the former President congratulated him on helping make the Apollo missions a success: “Without you, Tom, we would not have gotten to the moon”.
Later NASA spacecraft, such as the Space Shuttle (first launched in 1981), also made use of fuel cells as a key component of their electrical power system that could provide drinking water for the astronauts. These onboard fuel cells were capable of producing over 500 gallons of drinkable water on each shuttle flight – far more than all the astronauts could consume.
As time when on, scientific advancements in solar technology meant fuel cells fell out of favor as being the first choice to power spacecraft. This meant, however, that experts were now focusing on new uses for fuel cells. They were beginning to be seen as a viable solution to replace fossil fuel in transport vehicles.
Fuel Cell Cars, Mobile Phones and Drones: Continued Advancements, 1981- Present
Since the 1980s fuel cell development has increased at a brisk pace. While during the 1980s fuel cell stacks were still generally limited to space applications, the closure of the Apollo program resulted in many industry experts moving to private companies. By the 1990s fuel cell development was at such an advanced stage that the possibility of a fully functional fuel cell-powered car had the potential to become reality. This was only heightened by the development, in 2001, of the first 700 Bar (10000 PSI) hydrogen tank – meaning the size of fuel cell tanks were reduced enough to fit into vehicles for the first time.
In 2008, Honda finally released the first commercially available fuel cell car, the Honda FCX Clarity, the first hydrogen car available on the consumer market. As opposed to battery electric cars, this new consumer hydrogen car could be refueled in as little as 5 minutes while also having a long-range.
From 2008 to 2014, over 20 other fuel-cell electric vehicle prototypes were released, including the GM HydroGen4 and Mercedes-Benz F-Cell, and today over 56,000 hydrogen fuel-cell cars have been sold.
During the last two decades, companies have also been experimenting with fuel cell-powered mobile phones, forklifts, delivery drones, airplanes and long-haul trucks.
The prospect of a hydrogen-powered fuel cell drone is one area where recent advancements in the technology have the potential to revolutionize an entire industry. Current drones are significantly hindered by the power and range provided by conventional batteries. The average consumer drone, for example, has a flight time of 20-30 minutes.
Fuel cells offer the potential to overcome this challenge – boosting the range of drones by up to three times of battery-powered systems. A recent prototype even proved itself as being able to fly up to 5 hours without refueling.
Much of these new uses for fuel cells are the result of innovation resulting from increased research and development (R&D). Recent developments in nanotechnologies have brought about the possibility of less expensive fuel cell cathodes (which are currently made out of platinum). New manufacturing techniques for electrodes and catalysts are resulting in improved water management, and new fuel cell monitoring systems and sensors will mean improved reliability and lifespan.
Due to these advancements, fuel cells are widely expected to play a major role in the international commitment to net zero emissions by 2050.
Conclusion
Compared to the days of Anthony Carlisle and William Grove, today’s fuel cells are unrecognizable. But while fuel cells have transformed from containing a simple foil immersed in oxygen and hydrogen to advanced systems with monitoring sensors and materials from nanotechnology – their fundamental structure remains unchanged over 200 years. While the current technology inside fuel cells is advanced enough to power new (and unusual) applications, such as drones, airplanes and forklifts – they still contain the same basic elements (two electrodes sandwiched around an electrolyte) as their predecessors two centuries ago.
This concept (using hydrogen and oxygen to produce electricity with water and heat generated) has progressed from the small, exploratory experiments of Anthony Carlisle and William Grove to advanced, state-of-the-art systems capable of powering an array of applications. These modern-day fuel cells and their applications – new hydrogen-powered cars, trains, busses, drones and even airplanes and boats – have the potential to decarbonize global energy production and help nations around the world meet a commitment to net zero emissions by 2050.
Interested in learning more about fuel cells and fuel cell stacks? Check out Horizon Educational’s article on 8 Types of Fuel Cell by Price Point.

