The Amazing Technology Inside Fuel Cell Stacks
Have you ever wondered what exciting kinds of technology are inside fuel cell stacks? From electrolytes to anodes and cathodes – we’re here to tell you everything you need to know about this fascinating future technology that promises to decarbonise global energy.
What’s a Fuel Cell Electrolyte?
Every fuel cell stack must – by definition – have an electrolyte. This component of a fuel cell performs one of its most essential functions: carrying the electrically charged particles from one part of the fuel cell to the other. According to researchers from The Electrochemical Society, an electrolyte can be made of anything from potassium hydroxide to salt carbonates to phosphoric acid.
The particles the electrolyte carries take different paths to the other side of the fuel cell (the cathode). The electrons go through an external circuit, creating an electrical current, while the protons move through the electrolyte and reach the cathode, where they combine with oxygen and the electrons to create water and heat.
With the only by-products produced being water and heat, fuel cell stacks are one of the cleanest sources of energy on Earth.
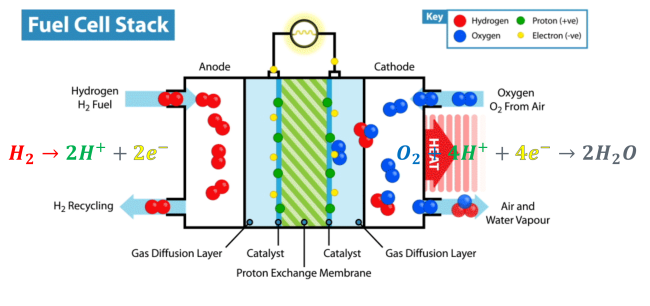
What’s a Fuel Cell Anode?
For the fuel cell to send protons and electrons to different parts of the system, these need to be chemically separated. This is where the fuel cell’s anode comes into play. Anodes do this incredible job with the help of a catalyst (catalysts help separate the hydrogen molecules without undergoing any permanent chemical changes themselves).
The substance engineers use as an anode’s catalyst is usually platinum, but researchers from the University of Western Cape have found that modified titanium and silicon dioxide can be used to improve the electrical charge, mass transfer resistance, power density and exposure of the catalyst to the hydrogen particles. This could mean more clean electricity produced from cheaper fuel cell stacks.
What’s a Fuel Cell Cathode?
The cathode is where the magic happens. Drawing electrons from the anode and protons from the electrolyte, the cathode then mixes them together again to create water and heat.
The cathode plays a vital role in every fuel cell – drawing the electrons through the system that create the electrical current. It’s important to note there are several factors that can influence the effectiveness of the cathode and the fuel cell, such as temperature, humidity, pressure, and cathode materials and design.
Researchers at the Ulsan National Institute of Science and Technology in Korea are currently examining more efficient materials that can be used in cathodes – such as manganese oxide-hydroxide or perovskite – that can help some fuel cells operate at a lower temperate and thus become more reliable and stable throughout their lifecycles.
Conclusion
While the current technology inside fuel cells is fascinating – future advancements will mean more efficient, reliable and cheaper fuel cell stacks. Many businesses are already investing heavily in hydrogen and fuel cell technology – and with fuel cell prices getting lower by the month, buying a fuel cell is beginning to look like an increasingly wise investment.
Interested in learning more about fuel cell stacks and their technology? Check out our Ultimate Guide to Fuel Cells in 2023.

